Media centre

In Australia, 1-in-9 new bowel cancer cases (1,716) occur in people under the age of 50 each year. Bowel cancer is the second most common cancer in women after breast cancer.
Bowel cancer treatment can come with fertility risks and understanding the options for preservation of fertility is an important consideration for many patients.
Just as all other side effects are discussed, possible impacts on reproductive function and fertility should be part of any discussion with your treating specialist before starting treatment for bowel cancer.
- Details
A key five-year Bowel Cancer Australia campaign has reached a milestone with updated clinical practice guidelines lowering the bowel cancer screening start age recently endorsed by the National Health and Medical Research Council (NHMRC).
For the first time, population screening (for people at average risk of developing bowel cancer, i.e., without any symptoms) is recommended every two years for people aged 45-74 (previously 50-74), along with a lowering of the National Bowel Cancer Screening Program (NBCSP) start age from 50 to 45.
People aged 40-44 (previously 45-49) are also able to request screening via their healthcare professional prior to receiving their first NBCSP invitation.
- Details
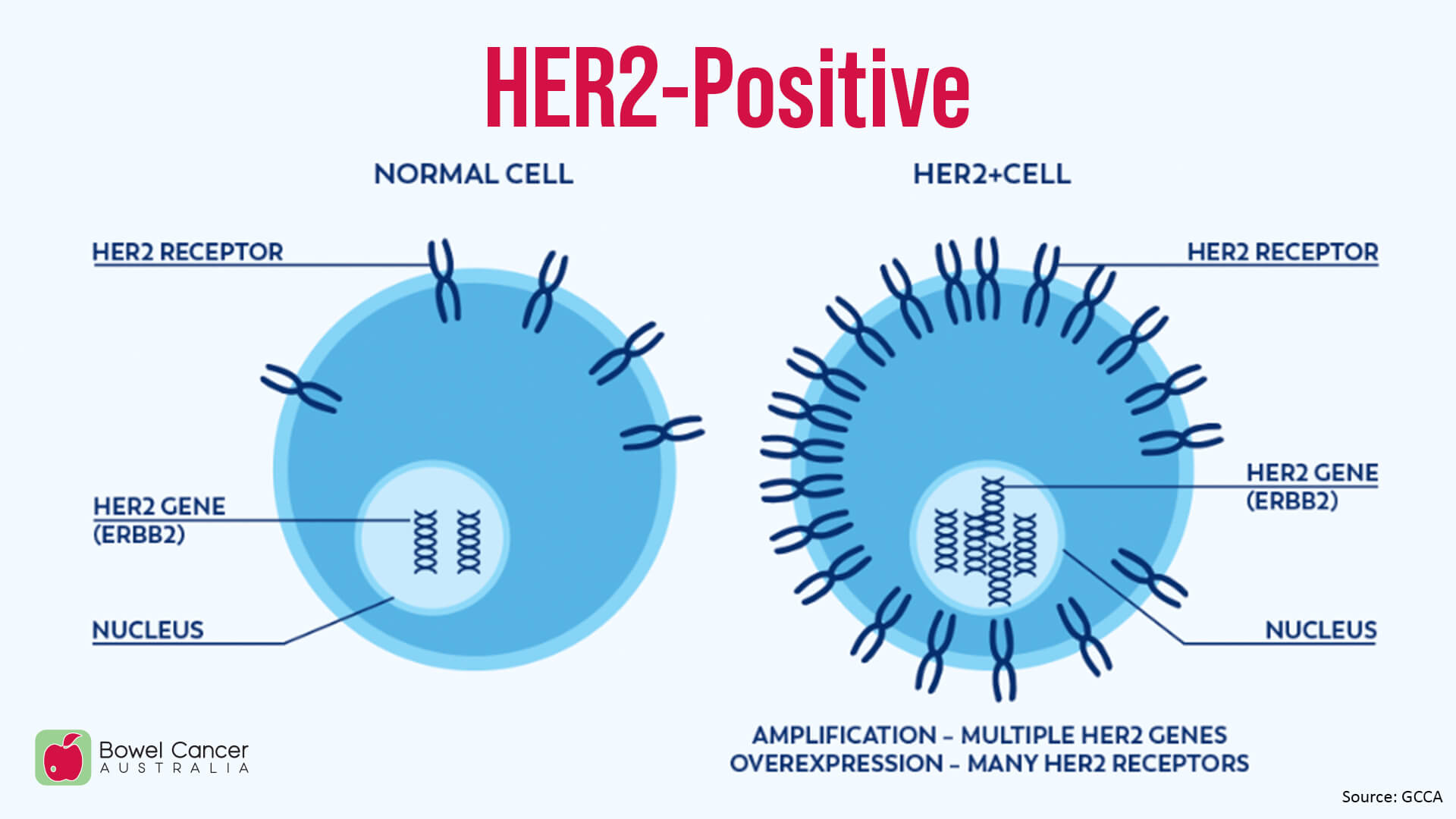
HER2 (also known as ERBB2), human epidermal growth factor receptor-2, is a gene that plays a role in the control of cell growth and cell survival.
Some cells may have an increased number of copies of the HER2 gene (overamplification) which can cause the cells to make too many HER2 receptors (overexpression). When these changes occur, they allow abnormal cell growth and survival.
- Details
Did you know vacuuming the house or running after the kids could lower your risk of some cancers?
New research led by the University of Sydney reveals the potential benefits of vigorous incidental activity. The study suggests a total of just 4.5 minutes of vigorous activity that makes you huff and puff during daily tasks could reduce the risk of some cancers by up to 18 percent and up to 32 percent for cancers linked to physical activity.
- Details
On 1 November 2019, a number of changes to colonoscopy and related items came into effect, resulting in eight new MBS items and the removal of four existing items.
The new Medicare item numbers for colonoscopy were the most dramatic change since they were created, and while there are no restrictions for people accessing colonoscopy if they have new symptoms or a positive screening test result, many patients with a personal or family history of the disease have been affected, resulting in stress and anxiety.
- Details
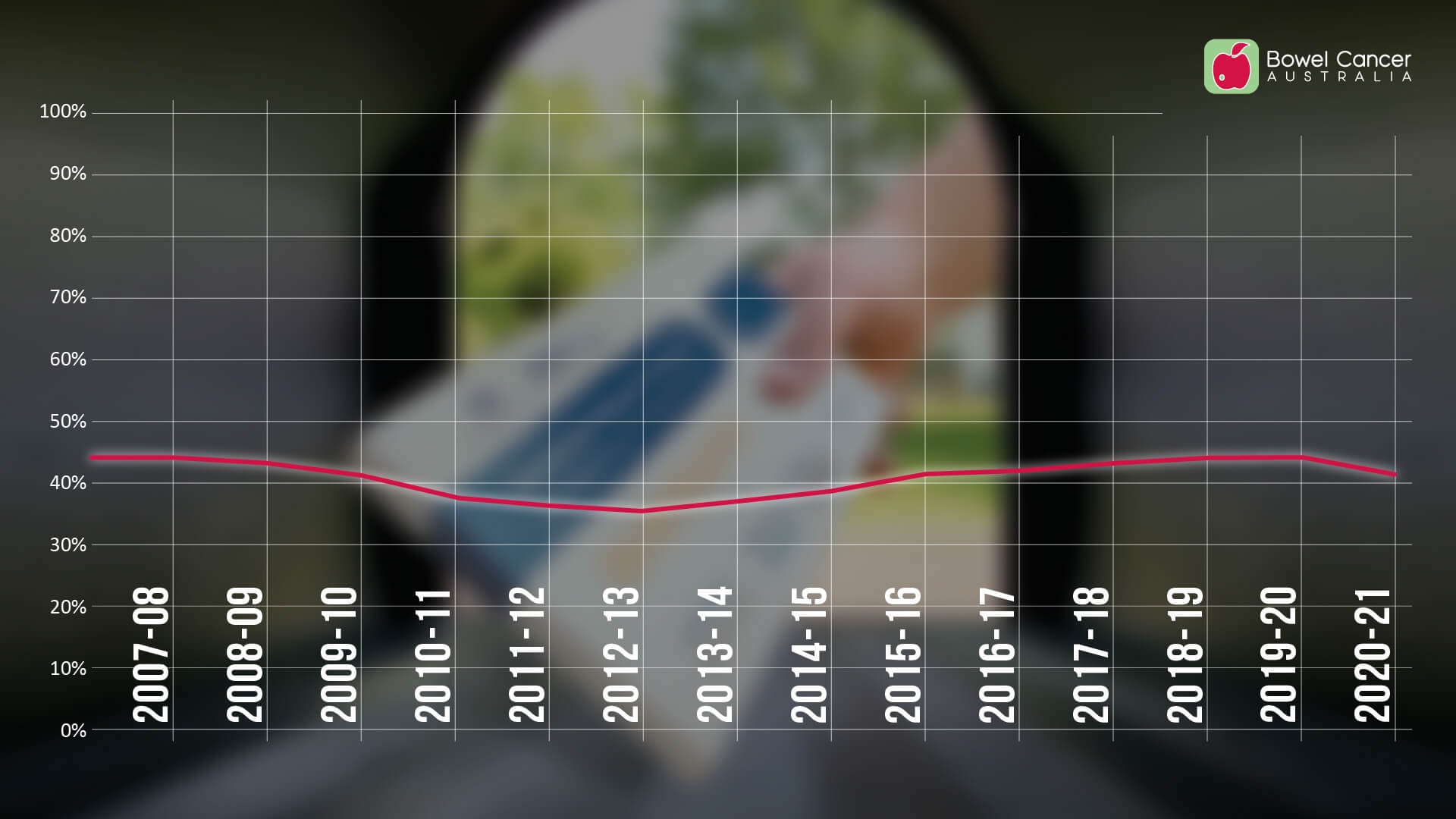
The latest AIHW National Bowel Cancer Screening Program (NBCSP) Report (2020-21) reveals participation rates in the Government program have fallen for a second time within a decade from 43.8% (2019-20) to 40.9% (2020-21).
The current participation rate is now the same rate as it was in 2015-16.
- Details
Founded on the belief that success means nothing unless you’re giving back, Francesca Jewellery combines beautiful design with an empowering purpose.
Francesca is supporting Bowel Cancer Australia for its fourth year with their Awareness Bracelet campaign. $20 from every bracelet purchased is donated to support those affected by bowel cancer.
- Details
Grampians Health is delighted to welcome a new dedicated Bowel Care Nurse, a position funded by Bowel Cancer Australia to provide tailored support to bowel cancer patients across the region.
Jaymee Goldsmith, a registered nurse who has specialist knowledge and experience caring for patients with bowel cancer, will be working two days a week at Ballarat Base Hospital and St John of God Hospital Ballarat to support bowel cancer patients across the Grampians region and assist them in their treatment journey.
- Details
Bowel Cancer Australia launched the final instalment of its bold 'Give a $#*! About Your Bowel' advertising campaign featuring acclaimed actress Miriam Margolyes OBE in the lead up to Bowel Cancer Awareness Month 2023.
The third instalment of this high impact series focuses on 'cancer' with a clear message that when caught early almost 99% of bowel cancer cases can be successfully treated.
- Details