APC | BRAF | HER2/ERBB2 | KRAS | MSI-H/dMMR | NRAS | NTRK fusion | PIK3CA | TP53 | RAS biomarker testing | FAQs
You are unique. So is your tumour.
That means your tumour might not respond to the same treatment that another bowel cancer patient receives.
By identifying your tumour's unique genes, biomarker testing helps you and your treatment team develop a personalised treatment plan just for you.
Cancer biomarkers are markers, changes and mutations in genes, DNA or proteins that are found in blood, tissue, or other body fluids and give us valuable information about the characteristics of a tumour.
Testing for specific biomarkers depends on several factors, including your age at diagnosis, stage of cancer, and overall health.
Even if your bowel cancer is not curable by surgery, tailored treatment can prolong survival and improve quality of life.
Biomarker testing is done by analysing biopsies to identify gene mutations. Molecules may be obtained from your blood, body fluids, or tissue.
Ideally, biomarker testing should be done before you start treatment as it may impact on your treatment plan, but it can still be done after treatment has commenced.
After a biopsy is taken, a pathologist analyses it and looks for abnormalities, then reports back to your treatment team.
The type of test(s) done may be limited to looking at a few genes or involve a multi-gene panel test or NGS (Next Generation Sequencing).
There are different types of biomarker testing, including:
- Single biomarker tests
- Multigene or panel tests: look for multiple biomarkers at the same time
- Whole-exome sequencing: looks at all the genes in your cancer
- Whole-genome sequencing: looks at all the DNA in your cancer
- Tumour-mutational burden (TMB): examines the extent/amount of genetic change in your cancer, and can help to determine if a type of immunotherapy known as immune-checkpoint inhibitors may work for you
A biomarker test result can be negative/wild-type (as it most commonly occurs in nature) or positive/mutant (with a mutation).
Speak with your specialist about what biomarker tests are available immediately after your diagnosis and before you agree on a treatment plan. If you are already on treatment and are unsure whether you have received biomarker testing, ask your treatment team.
New knowledge and improved testing has shown that additional mutations are also implicated in cancer resistance to certain treatments and responsiveness to others.
Certain biomarkers can also indicate progression of disease, so it’s important to discuss biomarker testing as an ongoing part of your treatment plan.
There are seven distinct biomarker types, each with its own specific function:
- Susceptibility or risk biomarker: indicates the potential for developing a condition or disease
- Diagnostic biomarker: detects or confirms the presence of a condition, disease or disease sub-type
- Monitoring biomarker: assesses the status or progression of a condition or disease
- Prognostic biomarker: identifies the likelihood of disease progression, disease recurrence or a clinical event
- Predictive biomarker: predicts response to treatment (likelihood of experiencing a favourable or unfavourable effect)
- Pharmacodynamic biomarker: demonstrates a biological response has occurred after treatment
- Safety biomarker: measures the likelihood, presence or extent of toxicity after treatment
Biomarkers can fit into more than one category, for example a biomarker can have both a prognostic and a predictive value.
Personalising (or tailoring) medical treatment according to your biomarkers can help you to avoid potential adverse effects and unnecessary costs related to treatments shown to be ineffective, or avoid delay in seeking alternative treatments which may be effective.
Knowing your biomarker status can also help your treatment team identify the best treatment options available, based on your tumour’s genetic makeup, and can help determine if you’re eligible for any clinical trials.
Bowel cancers arise through a series of mutations that occur over the space of many years and result in the evolution of normal tissue to adenoma (polyp) to carcinoma (cancer) to metastasis.
Biomarkers specific to bowel cancer, in order of prevalence, are:
- TP53 (58%)
- APC (57%)
- KRAS (39% colon, 37% rectal)
- PIK3CA (19% colon, 10% rectal)
- BRAF (12% colon, 4% rectal)
- MSI-H/dMMR (5% of metastatic)
- HER2 (5%)
- NRAS (4%)
- NTRK (3%)
In bowel tumours, the most common mutations occur in the TP53, APC, KRAS, PIK3CA and SMAD4 genes.
The most frequent predictive biomarkers currently used for bowel cancer treatment are the BRAF and RAS (KRAS and NRAS) gene mutations, but less frequent mutations can also inform treatment decisions.
The most frequent predictive biomarkers currently used for bowel cancer treatment are the BRAF and RAS (KRAS and NRAS) gene mutations, but less frequent mutations can also inform treatment decisions.
The latest research and treatments across many cancer types (including bowel cancer) can be found at My Cancer Genome, a website that includes a database of information on genes, genetic mutations, and cancer.
BRAF is altered in 12.45% of colon cancer patients with BRAF V600E present in 9.19% of all colon cancer patients.
BRAF is altered in 4.02% of rectal cancer patients with BRAF V600E present in 1.57% of all rectal cancer patients.
BRAF is a predictive biomarker for use of Erbitux (cetuximab), Vectibix (panitumumab) and Braftovi (encorafenib) in patients.
ERBB2 is altered in 4.69% of bowel cancer patients with ERBB2 Amplification present in 2.22% of all bowel cancer patients.
Patients with KRAS wild-type or NRAS wild-type (no mutation present), are more likely to have ERBB2 Amplification.
Enhertu (trastuzumab deruxtecan), Tykerb (lapatinib), Herceptin (trastuzumab), and Perjeta (pertuzumab) have evidence of efficacy in patients with ERBB2 mutation and ERBB2 Amplification in bowel cancer.
KRAS is mutated in 39.02% of colon cancer patients and 36.83% of rectal cancer patients.
MSI-H/dMMR constitute around 5% of metastatic bowel cancer cases.
MSI-H/dMMR is a predictive biomarker for use of nivolumab (Opdivo), pembrolizumab (Keytruda) and fluorouracil (5-FU) in patients.
NRAS is mutated in 4.19% of colon cancer patients and 4.41% of rectal cancer patients.
NRAS is a predictive biomarker for use of Erbitux (cetuximab) and Vectibix (panitumumab) in patients.
| NTRK (Neurotrophic tyrosine receptor kinase) gene fusion
| NTRK (Neurotrophic tyrosine receptor kinase) gene fusion
There are three NTRK genes (1, 2, and 3) that contain the information for making three similar proteins: TRK-A, TRK-B, and TRK-C.
It is rare, but the NTRK genes can fuse with other non-related genes which then produce new NTRK fusion proteins that promote uncontrolled cell growth and division in cancer cells.
NTRK1 is altered in 3.35% of bowel cancer patients. NTRK2 is altered in 2.31% of bowel cancer patients. NTRK3 is altered in 3.77% of bowel cancer patients.
NTRK fusion proteins are seen more commonly in microsatellite instability (MSI-H) tumours.
NTRK is a predictive biomarker for Vitrakvi (larotrectinib) and Rozlytrek (entrectinib).
PIK3CA is mutated in 19.51% of colon cancer patients and 10.19% of rectal cancer patients.
PIK3CA is a predictive biomarker for chemotherapy (FOLFOX, CAPOX or FOLFIRI) with or without Avastin (bevacizumab).
Patients with PIK3CA do not respond well to EGFR inhibitors - Erbitux (cetuximab) or Vectibix (panitumumab).
TP53 is mutated in 58.35% of bowel cancer patients.
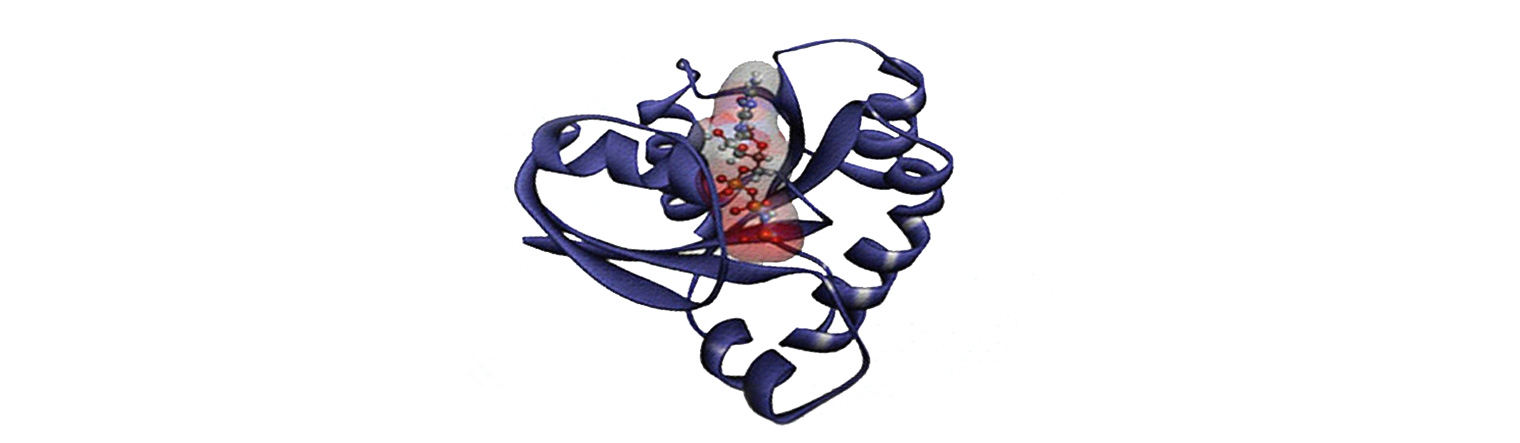
Human KRAS protein. (Image by Sarangan Ravichandran, Ph.D., Frederick National Laboratory for Cancer Research). Source: National Cancer Institute
| What is RAS?
The RAS gene family – which includes KRAS, NRAS and HRAS - produces proteins (RAS proteins) that are involved in cell signalling.
RAS proteins play an important role in the regulation of cell growth, cell division and cell death.
RAS genes enable cells to grow normally and everyone has them.
Normal RAS genes are also called 'wild-type' RAS genes.
Some people will develop an abnormal or 'mutated' version of the RAS gene.
Mutated RAS genes result in RAS proteins being locked into a permanently 'switched on' state. Like a car with an accelerator that won't release and brakes that won't engage, cell growth and divisions become out of control and dodge signals to die.
RAS mutations can also allow cells to resist available targeted therapies.
RAS proteins play an important role in the regulation of cell growth, cell division and cell death.
RAS genes enable cells to grow normally and everyone has them.
Normal RAS genes are also called 'wild-type' RAS genes.
Some people will develop an abnormal or 'mutated' version of the RAS gene.
Mutated RAS genes result in RAS proteins being locked into a permanently 'switched on' state. Like a car with an accelerator that won't release and brakes that won't engage, cell growth and divisions become out of control and dodge signals to die.
RAS mutations can also allow cells to resist available targeted therapies.
Why is RAS status important?
RAS status is important as it gives your oncologist the information they need to decide if adding a targeted therapy to your chemotherapy treatments may work for you. It does not affect the way your chemotherapy is prescribed.
About half of metastatic bowel cancers will have RAS 'wild-type' genes and about half will have 'mutated' RAS genes.
Patients with 'wild-type' RAS genes may respond to treatments with the targeted therapies Erbitux (cetuximab) or Vectibix (panitumumab). It is therefore important to know the RAS status of a cancer before your treatment commences.
About half of metastatic bowel cancers will have RAS 'wild-type' genes and about half will have 'mutated' RAS genes.
Patients with 'wild-type' RAS genes may respond to treatments with the targeted therapies Erbitux (cetuximab) or Vectibix (panitumumab). It is therefore important to know the RAS status of a cancer before your treatment commences.
Patients with 'mutated' RAS genes are unlikely to benefit from Erbitux (cetuximab) or Vectibix (panitumumab).
If this is the case, patients may still benefit from targeted therapies which work in a different way such as Avastin (bevacizumab).
When should I be tested?
RAS testing is recommended as soon as you are diagnosed with metastatic bowel cancer.
Do I need another biopsy or more surgery to provide the sample for testing?
Initial RAS testing can be performed on samples stored from the original surgery. Those samples may have been frozen or fixed in formalin.
Will I only need one RAS test?
There is evidence that some people will develop acquired resistance to EGFR inhibitors. A RAS test may need to be repeated if the treatment is no longer working and/or the cancer progresses.
Is RAS testing expensive?
RAS testing is reimbursed by Medical Benefits Scheme; Item No: 73338.
RAS testing is recommended as soon as you are diagnosed with metastatic bowel cancer.
Do I need another biopsy or more surgery to provide the sample for testing?
Initial RAS testing can be performed on samples stored from the original surgery. Those samples may have been frozen or fixed in formalin.
Will I only need one RAS test?
There is evidence that some people will develop acquired resistance to EGFR inhibitors. A RAS test may need to be repeated if the treatment is no longer working and/or the cancer progresses.
Is RAS testing expensive?
RAS testing is reimbursed by Medical Benefits Scheme; Item No: 73338.