Biomarker testing identifies the unique mutations in your tumour, helping your medical team develop a treatment plan that is tailored to you, more efficient, and less likely to provide unnecessary side effects.
Dying tumour cells release small pieces of their DNA into the bloodstream. These pieces are called circulating tumour DNA (ctDNA).
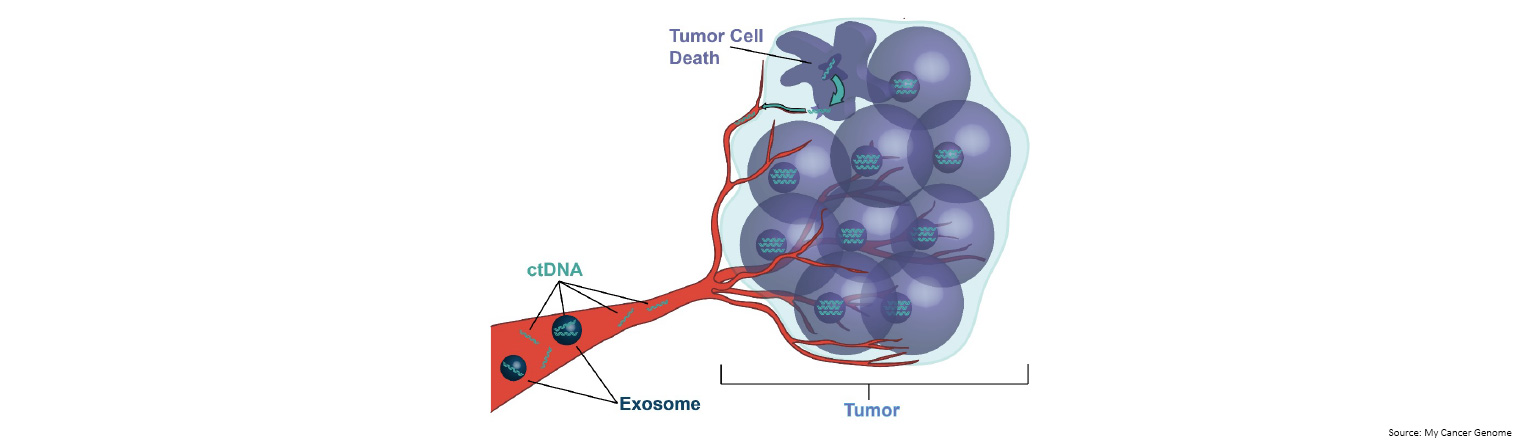
A ‘liquid biopsy’, collected via a sample of blood or other bodily fluid, can be performed to detect ctDNA and identify specific mutations (including biomarkers) that may have prognostic and/or therapeutic implications.
CtDNA has various clinical applications, including molecular profiling, treatment response prediction, real time monitoring of treatment response, early assessment of recurrence risk through the detection of minimal residual disease (MRD), and real time monitoring for disease recurrence.
One of its notable advantages is its ability to rapidly provide insight into a patient’s response to therapy, in real time, giving specialists more agility in their adjustment of treatment plans. Therapies can be escalated, de-escalated, or ceased, and new therapies introduced, depending on a patient’s response.
CtDNA has also been shown to predict recurrence of bowel cancer with a median lead time of 8.7 months before radiographic assessment.
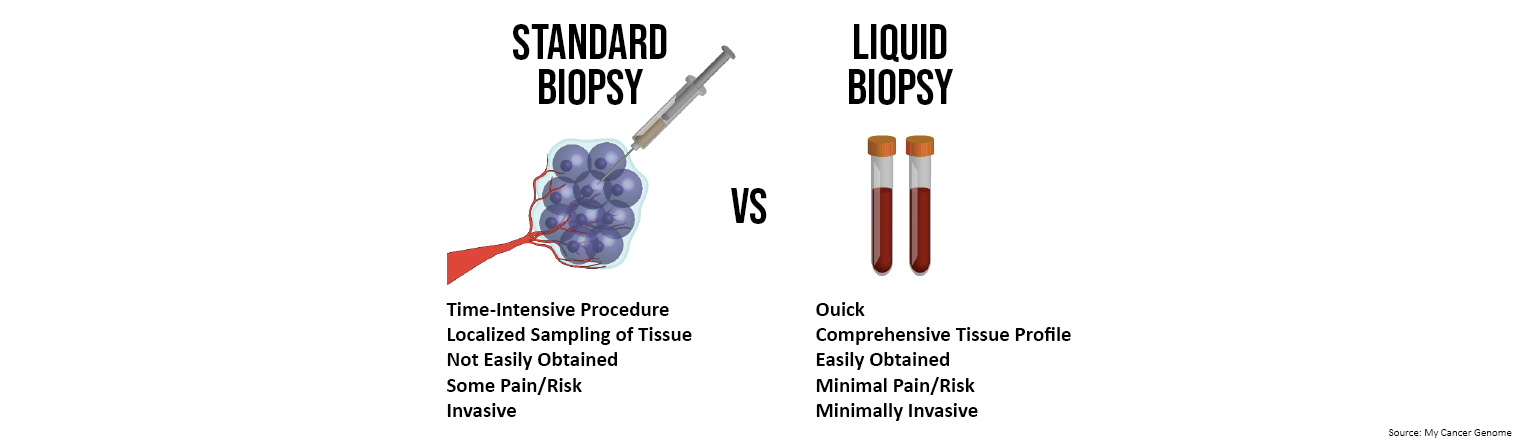
Collection of ctDNA via liquid biopsy is quick, convenient, minimally invasive, and provides a comprehensive profile of both primary and metastatic lesions. Analysis of the resected primary tumour alone via tissue biopsy, which is current standard practice, may provide a misleading representation of metastatic lesions.
Metastases can develop unique characteristics that may not be detected when assessing the primary tumour alone.
While CtDNA shows promise for patients with metastatic bowel cancer, its use also extends to earlier stage bowel cancer.
The DYNAMIC trial involving stage II bowel cancer patients is the first randomised study to provide clinical evidence of ctDNA’s role in reducing the need for adjuvant chemotherapy, without compromising patient survival. The findings are significant as previously there was no way of assessing whether all traces of a cancer had been removed following surgery.
“Our trial has conclusively shown how the ctDNA blood test can be used to direct post-surgical therapy in stage II colon cancer and substantially reduce the number of patients treated with chemotherapy, without impacting the risk of cancer relapse,” Associate Professor and DYNAMIC study author Jeanne Tie said.
“This ctDNA blood test could be used to spare around 600 Australians and over 100,000 people worldwide from unnecessary chemo treatments each year.”






