Anal cancer is considered a rare disease, with 514 people diagnosed and 129 deaths each year in Australia.
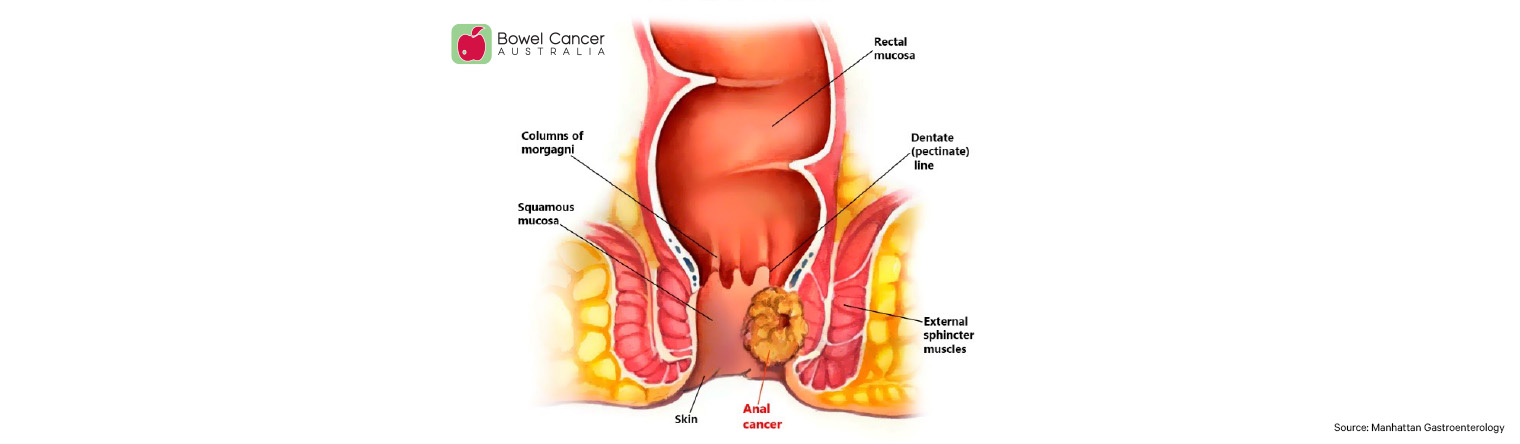
The anus (back passage) is the 4 cm long end portion of the large bowel, which opens to allow poo to exit the body. The anus is formed partly from the outer skin layers of the body and partly from the intestine. Two ring-like muscles, called sphincter muscles, open and close the anal opening and let poo pass out of the body.
In the general population, 90% of anal cancers are caused by a particular strain of the human papillomavirus (HPV), the most common sexually transmitted infection worldwide.
Anal HPV can lead to pre-cancerous cells called high-grade squamous intraepithelial lesions (HSIL) inside or around the anus in a similar way to cervical HPV causing pre-cancerous changes in the cervix.
However, unlike with cervical HSIL, there is currently no routine screening or treatment for anal HSIL.
The incidence of anal cancer is substantially higher in people living with HIV (PLWHIV) than in the general population. The rate of progression from anal HSIL to anal cancer isn’t known but it is thought it may be of the order of 1 in 4,000 in the general population and 1 in 100 in PLWHIV.
A new study, the Anal Cancer-HSIL Outcomes Research (ANCHOR) trial, led by the University of California San Francisco and published in the New England Journal of Medicine, is the first randomised control trial to demonstrate that treating anal HSIL is effective in reducing progression to anal cancer.
The phase 3 trial was conducted across 25 sites in the United States with 4,459 PLWHIV, aged 35 and older, who had biopsy-proven anal HSIL.
Participants were randomly assigned to two groups, one receiving treatment for anal HSIL, and one undergoing active monitoring of anal HSIL without treatment (a ‘watch and wait’ approach, which is the current standard of care in most countries).
The rate of progression to anal cancer was 57% lower in those who had received treatment for anal HSIL compared to those who just had active monitoring (without treatment).
The evidence was so compelling the trial was halted, and all patients in the ‘watch and wait’ group were transferred directly to treatment for anal HSIL.
Dr Penelope De Lacavalerie, colorectal surgeon and a member of the Australian Multidisciplinary Working Group for Anal Cancer Prevention in People Living with HIV, a nationwide group aiming to assess guidelines, treatment recommendations and implementation of research for screening for anal cancer in PLWHIV in Australia.
Currently, in this population, screening includes an anal pap smear for cytology and HPV typing and high resolution anoscopy (HRA) - similar to procedures used in the current cervical screening program in Australia.
She explains “there is no formal screening for anal cancer in Australia or worldwide. As the ANCHOR trial demonstrates, for PLWHIV it is now clear that treating HSIL is superior to a 'watch and wait' approach."
"The trial demands a response from the health system so that we can start anal screening in this high-risk group nationwide and prevent anal cancers and deaths."
"Screening of other high-risk groups is also likely to be of benefit - such as women with high-risk HPV infection or a history of genital cancer, those receiving solid organ transplants, and people with other immunosuppressing conditions such as inflammatory bowel disease. Ultimately, this will only be possible with adequate infrastructure and support from Medicare and the health system," she added.






