- reduce side effects
- extend survival, and
- limit toxicity exposure
These treatments may:
- stop cancer cells from dividing and growing
- seek out cancer cells and kill them
- encourage the immune system to attack cancer cells
- alter the growth of blood vessels into the tumour
Types of targeted therapies used in the treatment of bowel cancer including the following:
- Monoclonal antibodies: monoclonal antibodies are made in the laboratory from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attached to the substances and kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells.
There are different types of monoclonal antibody therapy:
-
- Vascular endothelial growth factor (VEGF) inhibitor therapy: cancer cells makes a substance called VEGF, which causes new blood vessels to form (angiogenesis) and helps the cancer grow. VEGF inhibitors block VEGF and stop new blood vessels from forming. This may kill cancer cells because they need blood vessels to grow. Bevacizumab is a VEGF and angiogenesis inhibitor.
-
- Epidermal growth factor receptor (EGFR) inhibitor therapy: EGFRs are proteins found on the surface of certain cells, including cancer cells. Epidermal growth factor attaches to the EGFR on the surface of the cell and causes the cells to grow and divide. EGFR inhibitors block the receptor and stop the epidermal growth factor from attaching to the cancer cell. This stops the cancer cell from growing and dividing. Cetuximab and panitumumab are EGFR inhibitors.
- Angiogenesis inhibitors: angiogenesis inhibitors stop the growth of new blood vessels that tumours need to grow.
-
- Aflibercept is a vascular endothelial growth factor trap that blocks an enzyme needed for the growth of new blood vessels in tumours.
- Regorafenib is used to treat bowel cancer that has spread to other parts of the body and has not gotten better with other treatment. It blocks the action of certain proteins, including vascular endothelial growth factor. This may help keep cancer cells from growing and may kill them. It may also prevent the growth of new blood vessels that tumours need to grow.
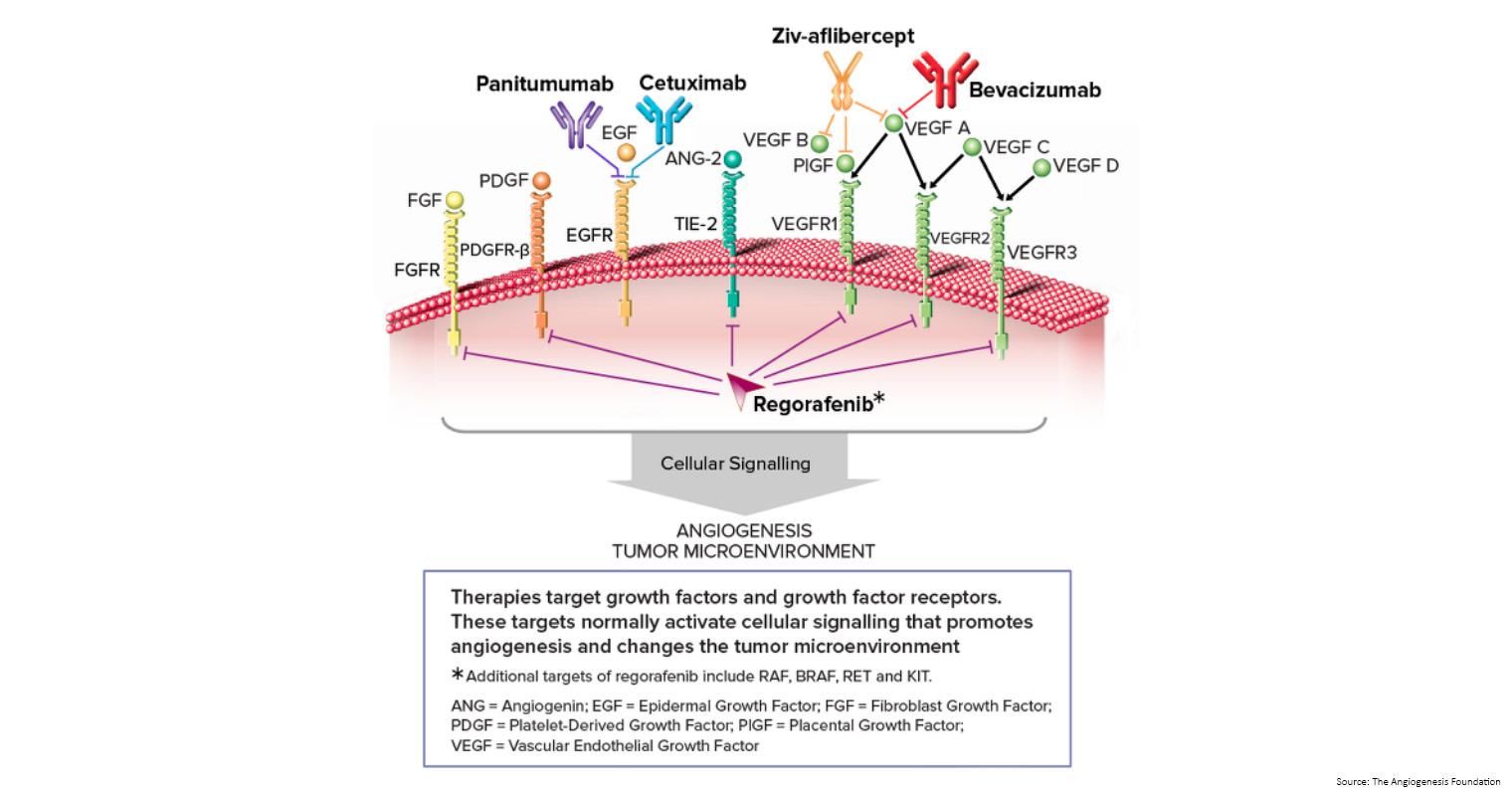
At what stage is this treatment used?
- Avastin had previously been approved by the Therapeutic Goods Administration (TGA) for use in Australia, however in December 2021 it was discontinued as a treatment option for patients with metastatic bowel cancer ‘due to the lack of demand for private purchase since it was removed from the Pharmaceutical Benefits Scheme (PBS) in June 2021. The manufacturer no longer supplies Avastin in Australia and it was removed from the Australian Register of Therapeutic Goods (ARTG). The ARTG is the register for all therapeutic goods that can be lawfully supplied in Australia. Sometimes a special provision is made to make available some medicines that are not listed in response to the needs of patients. To find out more visit the access to therapeutic goods on the ARTG website.
- Mvasi is a biosimilar medicine to Avastin and has been approved by the Therapeutic Goods Administration (TGA) and is registered on the Australian Register of Therapeutic Goods (ARTG) for use in Australia. Mvasi is PBS-subsidised for first- and second-line treatment of patients with metastatic bowel cancer.
At what stage is this treatment used?
- From 1 January 2022, encorafenib will be listed on the PBS as a subsidised treatment in combination with cetuximab for patients with BRAF V600E-variant metastatic bowel cancer who have not responded to a prior systemic therapy.
- Cetuximab is PBS-subsidised as a treatment in combination with first-line chemotherapy, of a patient with a WHO performance status of 0 or 1 and previously untreated RAS wild-type metastatic bowel cancer, who does not have progressive disease.
- Cetuximab is PBS-subsidised as monotherapy or in combination with first-line chemotherapy, of a patient with a WHO performance status of 2 or less and with RAS wild type metastatic bowel cancer after failure to respond to first-line chemotherapy. The treatment must be the sole PBS-subsidised anti-EGFR antibody therapy for this condition.
- Patients who have progressive disease on panitumumab are not eligible to receive PBS-subsidised cetuximab. Patients who have developed intolerance to panitumumab of a severity necessitating permanent treatment withdrawal are eligible to receive PBS-subsidised cetuximab.
- Regorafenib has been approved by the Therapeutic Goods Administration (TGA) and is registered on the Australian Register of Therapeutic Goods (ARTG) for use in Australia, however, it is not currently listed on the PBS as a subsidised treatment.
Download the Consumer Medicine Information (CMI) for Stivarga.
- Panitumumab is PBS-subsidised as a treatment in combination with first-line chemotherapy of a patient with a WHO performance status of 0 or 1 and previously untreated RAS wild-type metastatic bowel cancer, who does not have progressive disease.
- Panitumumab is PBS-subsidised as monotherapy or in combination with chemotherapy, of a patient with a WHO performance status of 2 or less and with RAS wild type metastatic bowel cancer after failure to respond to first-line chemotherapy.
- The treatment must be the sole PBS-subsidised anti-EGFR antibody therapy for this condition. Patients who have progressive disease on cetuximab are not eligible to receive PBS-subsidised panitumumab. Patients who have developed intolerance to cetuximab of a severity necessitating permanent treatment withdrawal are eligible to receive PBS-subsidised panitumumab.
At what stage is this treatment used?
Aflibercept (Zaltrap) is a type of targeted drug known as a fusion protein. This drug is designed to target multiple growth factors involved in cancer and angiogenesis, specifically proteins called VEGF-A, VEGF-B and a related protein called placental growth factor (PlGF).
Aflibercept is approved for use as treatment in second-line therapy in combination with chemotherapy (FOLFIRI) for metastatic bowel cancer.
Is this drug available via the Pharmaceutical Benefits Scheme (PBS) as a subsidised treatment?
- Aflibercept had previously been approved by the Therapeutic Goods Administration (TGA) for use in Australia, however in 2018 it was discontinued as a treatment option for patients with metastatic bowel cancer ‘due to cost effectiveness and other treatment options being more available’. The manufacturer no longer supplies Aflibercept in Australia and it was removed from the Australian Register of Therapeutic Goods (ARTG). The ARTG is the register for all therapeutic goods that can be lawfully supplied in Australia. Sometimes a special provision is made to make available some medicines that are not listed in response to the needs of patients. To find out more visit the access to therapeutic goods on the ARTG website.
What is cancer immunotherapy?
Immunotherapy is treatment that uses the patient's immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body's natural defences against cancer. This type of cancer treatment is also called biotherapy or biologic therapy.
- Monoclonal antibodies: These are man-made versions of immune system proteins. Antibodies can be very useful in treating cancer because they can be designed to attack a very specific part of a cancer cell.
- Immune checkpoint inhibitors: These drugs basically take the ‘brakes’ off the immune system, which helps it recognise and attack cancer cells.
- Cancer vaccines: Vaccines are substances put into the body to start an immune response against certain diseases. We usually think of them as being given to healthy people to help prevent infections. But some vaccines can help prevent or treat cancer.
- Other, non-specific immunotherapies: These treatments boost the immune system in a general way, but this can still help the immune system attack cancer cells.
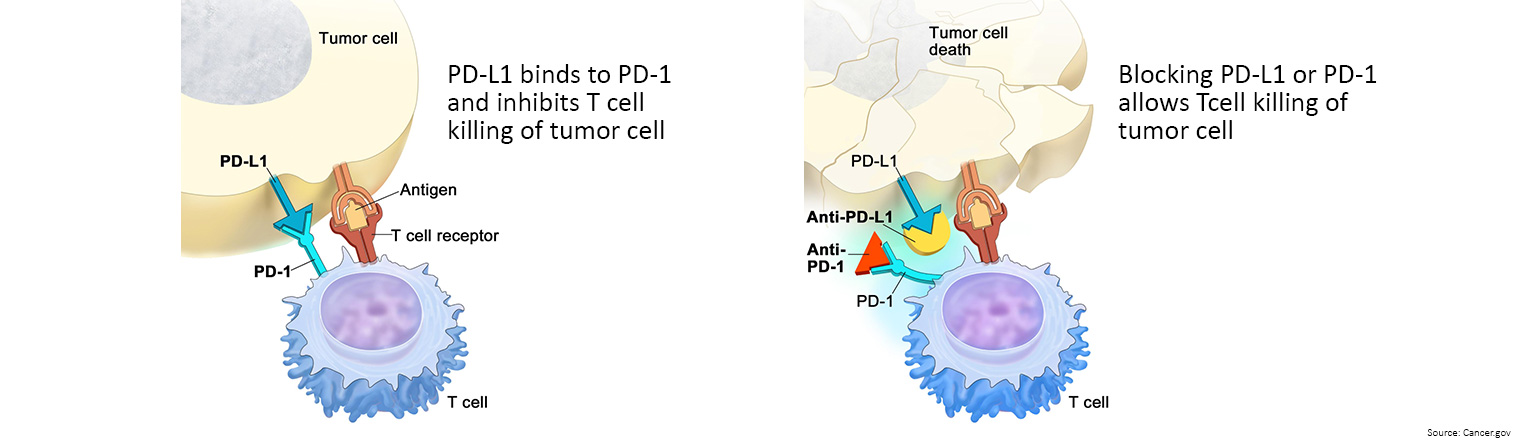
- Keytruda is currently listed on the PBS as a first-line treatment for previously untreated unresectable or metastatic deficient mismatch repair (dMMR) bowel cancer.
Download the Consumer Medicine Information (CMI) for Keytruda.
- Opdivo has not been approved by the Therapeutic Goods Administration (TGA) for use in Australia as a treatment option for bowel cancer, and is not currently listed on the PBS as a subsidised treatment. Sometimes a special provision is made to make available some medicines that are not listed in response to the needs of patients. To find out more visit the access to therapeutic goods on the ARTG website.
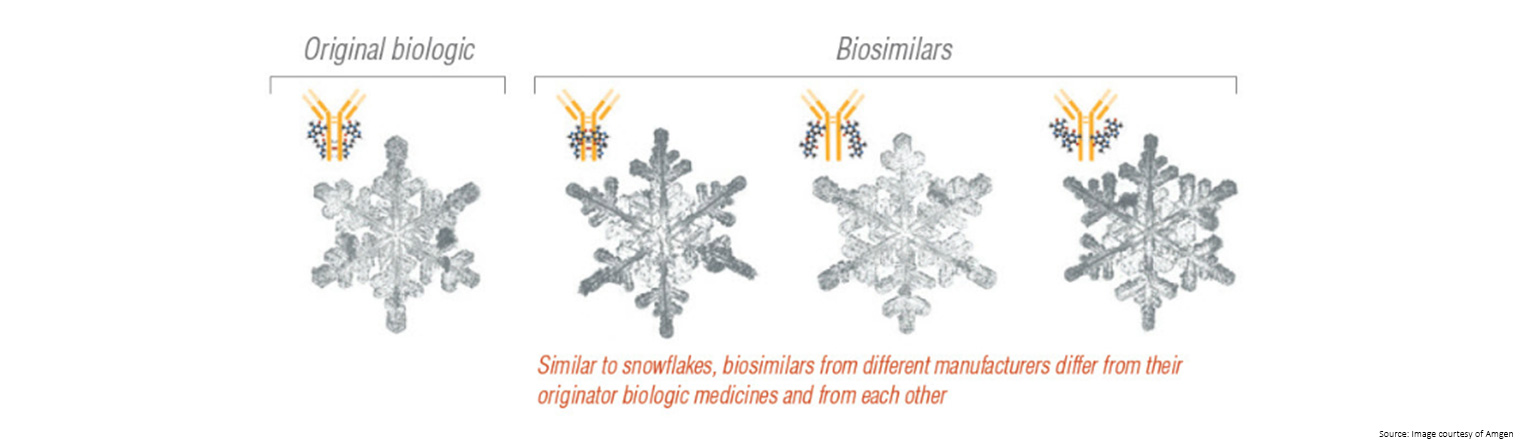
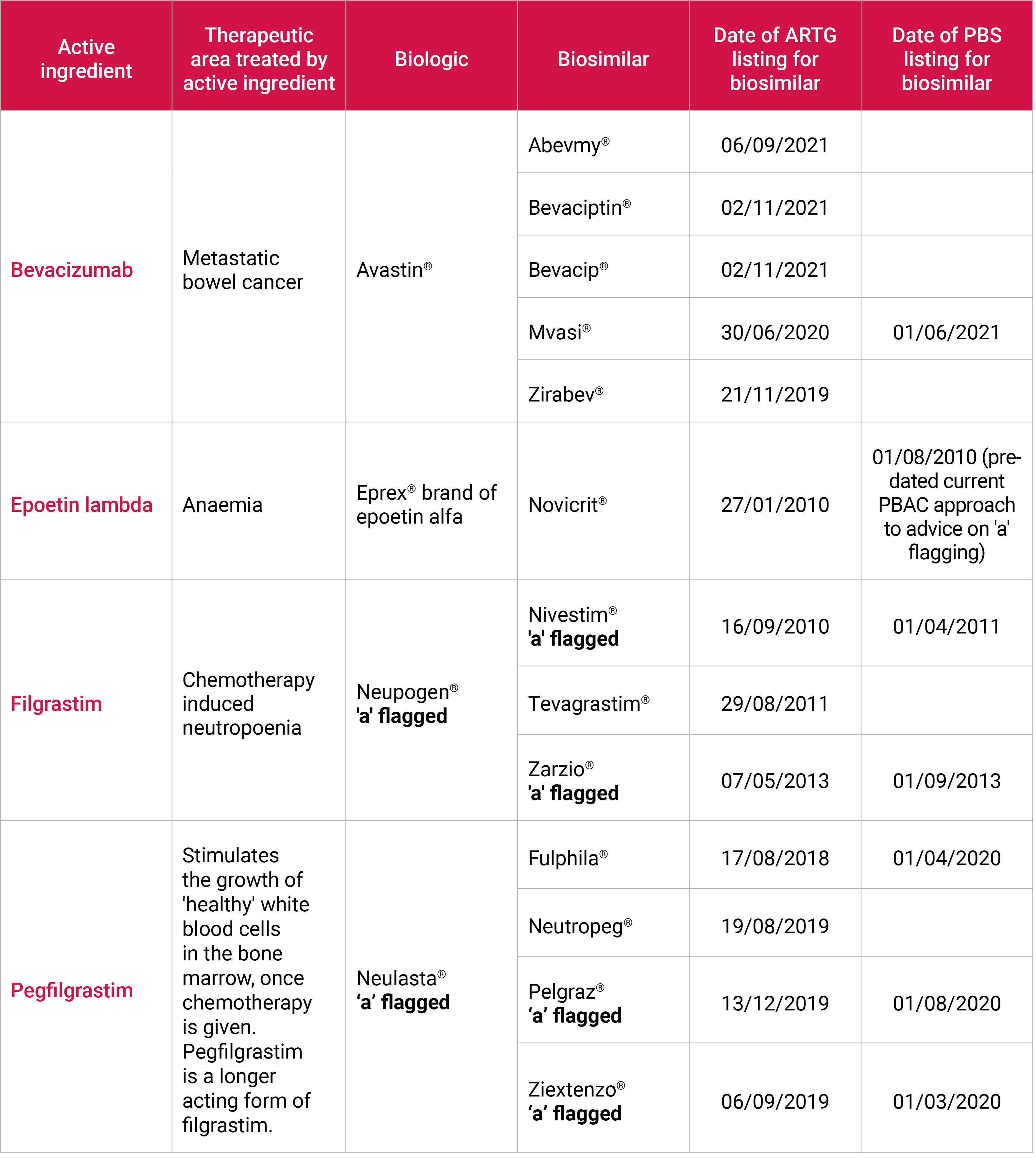
| Biosimilars
A medicine made from living organisms, such as protein, bacteria or yeast, is known as a biological medicine.
How biological and biosimilar medicines work
Unlike traditional chemical medicines which are made by combining chemical ingredients, biological and biosimilar medicines are made from living organisms.
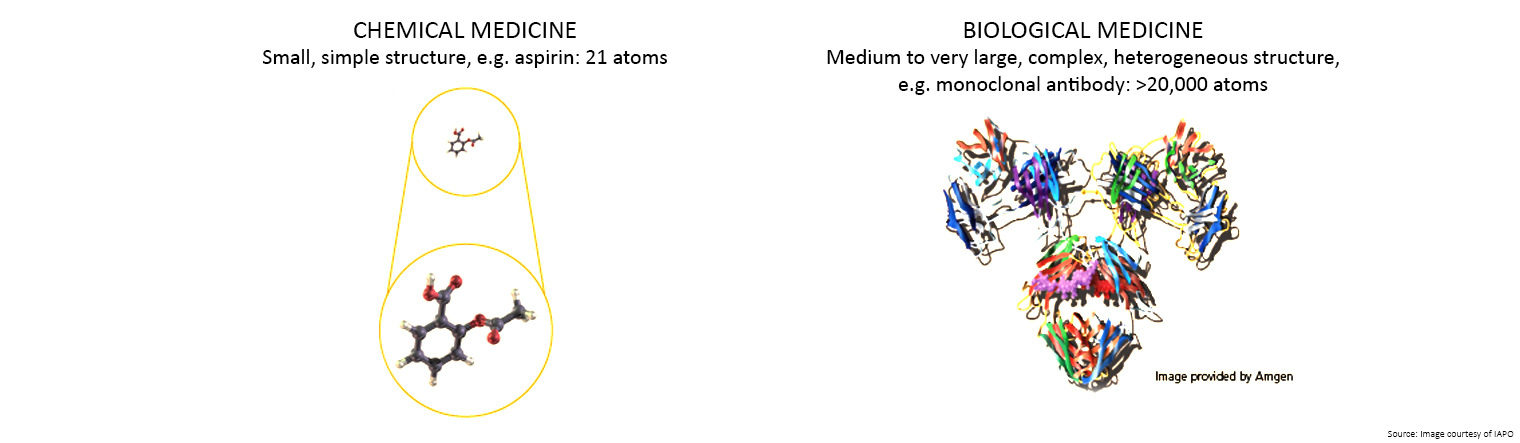
There are three main reasons why it is virtually impossible for different manufacturers to replicate a biological medicine:
- As living organisms, there will always be some differences between medicines even if this does not affect how the medicine works
- The structures are large and complex, making it difficult to create an exact copy
- The final medicine is highly dependent on the manufacturing process, and once the original patent for a medicine expires each manufacturer has to make their own decisions around the cell lines, conditions to grow the cells, stabilising compounds to use, as well as how the medicine is packaged and stored.
- Substitution - if two or more medicines are considered substitutable, the pharmacist may dispense either of these medicines from the script, provided the prescriber (i.e. GP or specialist) has not indicated ‘brand substitution not permitted’, and they have permission from the patient. This applies most often to generic medicines and biological medicines with a biosimilar that has been recommended by the Pharmaceutical Benefits Advisory Committee (PBAC) as substitutable. Substitutable medicines are marked in the Schedule of Pharmaceutical Benefits with an ‘a’ (a-flagged). It is important that the prescriber of the biologic is notified of the switch. This allows for appropriate monitoring of a patient's response to treatment , as well as tracking of adverse events.
- Interchangeability - if two or more medicines are considered interchangeable, the prescriber (i.e. GP or specialist) may choose to prescribe either of the medicines for a patient to treat the same condition; however, the pharmacist must dispense as prescribed. This generally occurs between two different medicines, rather than brands or biosimilars of the same medicine.
In 2017, the US Food and Drug Administration (FDA) issued draft guidance detailing the agency's expectations for demonstrating biosimilar interchangeability. To be approved as interchangeable, it must demonstrate that it 'can be expected to produce the same clinical result as the reference product in any given patient' when substituted, and 'the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch.'
- Switching between medicine brands could occur in either case. One would be at the direction of the prescriber (prescribing an interchangeable medicine), the other would occur at the pharmacy level (brand substitution).
What are the biosimilar uptake drivers?
As part of the 2017 Budget process the Government reached agreement with Medicines Australia, the Generic and Biosimilar Medicines Association and the Pharmacy Guild of Australia to implement biosimilar uptake drivers. Two specific biosimilar uptake drivers are being implemented:
- encouraging prescribing of a biosimilar brand rather than the reference biological brand for treatment naïve patients.
For the purpose of this uptake driver a patient is ‘treatment naïve’ if they have not previously taken the biological medicine intended to be prescribed. That is, they have not taken either the reference or biosimilar brand of the particular biological medicine.
Continued use of a biosimilar brand after it is first taken by a patient is a matter for the patient in consultation with their prescriber and pharmacist.
A patient is not treatment naïve if they took any brand of the particular biological medicine in the past, even if that treatment occurred some time ago.
- providing for a simpler and faster approval process for prescribing biosimilar brands (e.g. streamlined authority) while maintaining an existing higher level authority requirement for the reference biological brand (e.g. written authority).
There are three broad types of authority requirement for prescribing PBS medicines:
Written authority – the prescriber is required to obtain from the Department of Human Services an authority approval in writing for prescribing the medicine.
Immediate authority – the prescriber can get the authority to prescribe either online or by phone (which can occur during a consultation).
Streamlined authority – if the prescriber is satisfied that the specific eligibility criteria and rules for prescribing on the PBS are met, they mark a ‘streamlined authority code’ on the prescription. A ‘streamlined authority’ provides the simplest and fastest method for demonstrating authority to prescribe the medicine.
In the public hospital setting, brand decisions are made by clinician-led committees on behalf of doctors and patients.
Will the use of biosimilar medicines be forced?
In Australia, there is no current mandated requirement for GPs, pharmacists or patients to use biosimilar medicines.
By way of contrast, the Government of Alberta (Canada) has announced that it will begin forcibly switching 26,000 patients from their physician-chosen biologic medicines to government-chosen biosimilars beginning 1 July 2020. According to the Alliance for Safe Biologic Medicines (ASBM), only half of Albertan patients- those on public pharmacare plans- will be subject to the forced-switching policy. Patients on private health plans, including Ministry of Health officials, will not be forced to switch. Children and pregnant women are also exempted.
On 27 May 2019 the Government of British Columbia (BC, Canada) announced 20,700 patients would be forcibly switched from their existing medicines to the government’s choice of preferred biosimilar products and cease the reimbursement of their current biologic medicine. The policy disregards important scientific considerations for patients on biologic medicines and most alarmingly, eliminates patient choice, according to the Alliance for Safe Biologic Medicines (ASBM).
There is a growing number of biosimilar medicines available in Australia, and some of these are relevant to cancer patients receiving chemotherapy (see below).