Benefits & limitations | Colonoscopy | Virtual colonoscopy & MRI | Sigmoidoscopy | Barium enema | Faecal immunochemical test (FIT) | DNA stool test | Blood tests
If your GP suggests that you take tests or be referred to a specialist for further investigations, this does not mean you have bowel cancer.
It means that further tests and/or investigations are needed to determine the underlying cause of your symptoms.
If you receive a positive faecal immunochemical test result or have higher-risk symptoms such as blood in poo or rectal bleeding, you should receive an urgent referral and have a colonoscopy within 30 days.
It means that further tests and/or investigations are needed to determine the underlying cause of your symptoms.
If you receive a positive faecal immunochemical test result or have higher-risk symptoms such as blood in poo or rectal bleeding, you should receive an urgent referral and have a colonoscopy within 30 days.
If symptoms are not considered higher-risk, you will receive a routine referral.
The waiting list for routine referrals varies around Australia.
Further investigations will usually take place at a clinic in your local hospital.
The specialist will ask you questions about your symptoms (similar to questions asked by your GP), your general health and other medical conditions you might have.
You will sometimes be given the results from investigative tests immediately, or you will be called back to the hospital at a later date to receive the results.
If the further investigations for bowel cancer are negative, you may be diagnosed with another common gastrointestinal condition and given appropriate treatment.
If the further investigations confirm bowel cancer, you will meet with a specialist who will put together your treatment plan.
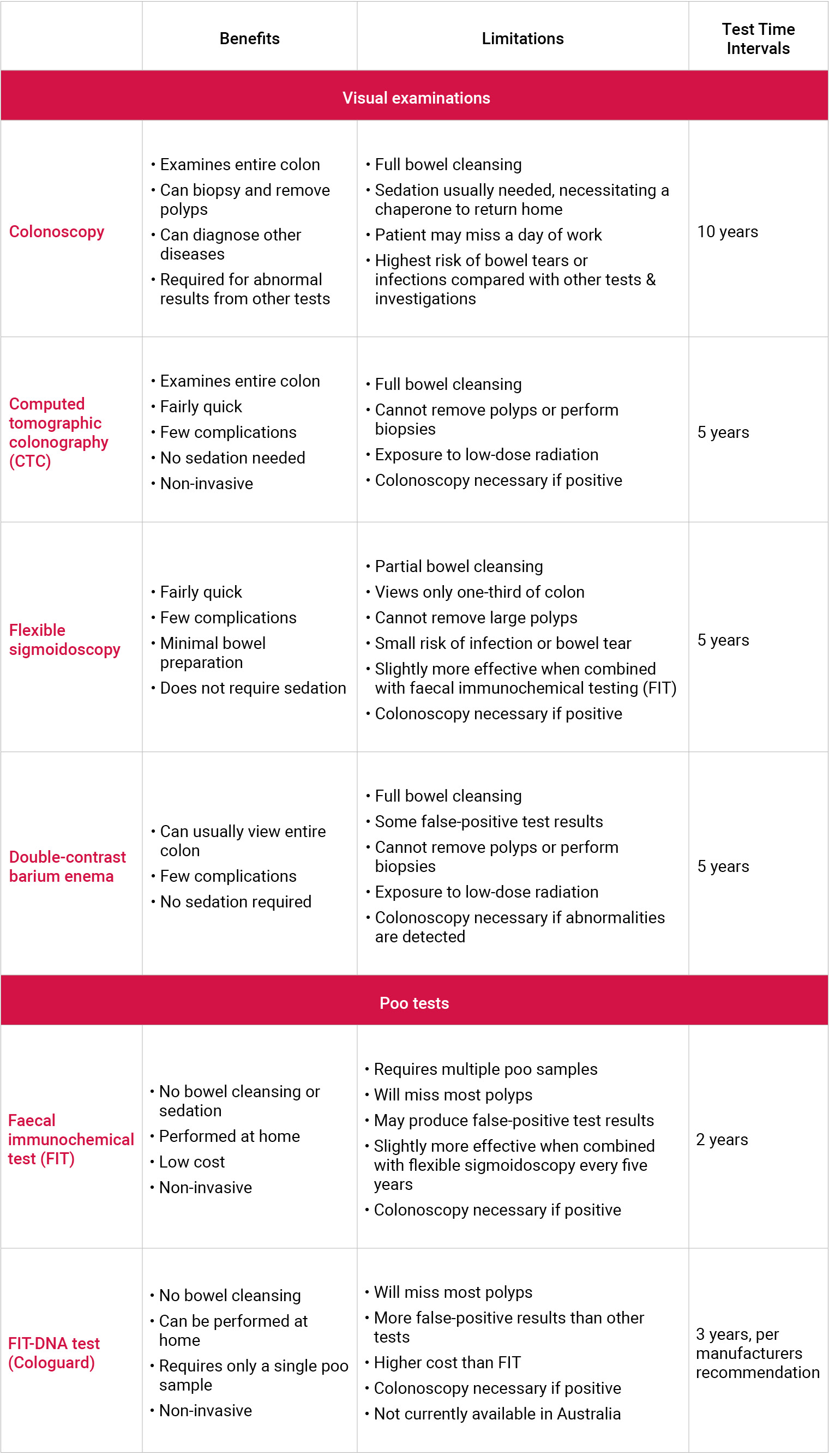
The following table details considerations when deciding with your GP and/or specialist what test is right for you.
| Benefits and limitations
Source: American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019. Atlanta: American Cancer Society; 2017.
| Colonoscopy
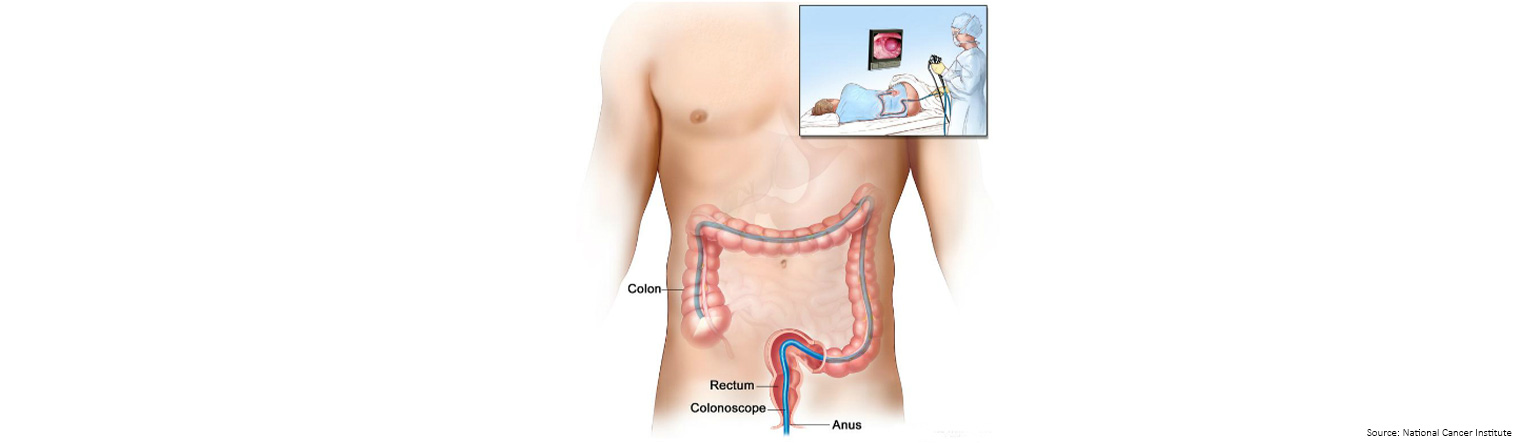
A colonoscopy is a quick and generally painless procedure that allows for the full examination of the entire inner lining of your bowel (colon and rectum).
During the procedure, the colonoscopist spends most of the time looking for changes to the normal landscape of your bowel lining and removes anything that looks suspicious, like growths called polyps.
Polyps are usually harmless (benign); they can be slightly raised (sessile), look like they are on a stalk like a cherry (pedunculated), or can be very flat.
Adenomatous polyps however, can become cancerous (malignant), and if left undetected can develop into a cancerous tumour.
Polyps can be detected and removed before they develop into bowel cancer during a colonoscopy, and bowel cancer, if present, can be diagnosed.
The colonoscopy usually lasts around 30 minutes or less and is typically performed under a general anaesthetic.
Because of the sedation, you should arrange for someone to collect you and take you home following the procedure.
Video-capsule colonoscopy
Video-capsule endoscopy has become an important tool for investigation of disorders of the small bowel. While there is interest in its potential for imaging the large bowel, the place for video-capsule colonoscopy is still uncertain.
| Virtual colonoscopy
Virtual colonoscopy (also known as Computerised Tomographic Colonography - CTC) is a procedure that uses a series of advanced imaging that permits minimally invasive evaluation of the colon and rectum without the need for sedation.
A computer puts the pictures together to create detailed images that may show polyps and anything else that seems unusual on the inside surface of the bowel.
If the detailed images show polyps and anything else that seems unusual and your specialist wishes to perform a biopsy, you will need to have a colonoscopy.
It has an established place in investigation of symptomatic patients and following incomplete colonoscopy.
The risk for procedure-related complications is low, although CT involves larger radiation doses than the more common, conventional x-ray imaging procedures.
A computer puts the pictures together to create detailed images that may show polyps and anything else that seems unusual on the inside surface of the bowel.
If the detailed images show polyps and anything else that seems unusual and your specialist wishes to perform a biopsy, you will need to have a colonoscopy.
It has an established place in investigation of symptomatic patients and following incomplete colonoscopy.
The risk for procedure-related complications is low, although CT involves larger radiation doses than the more common, conventional x-ray imaging procedures.
Several studies indicate that magnetic resonance colonography (MRC) could become an alternative to Computerised Tomographic Colonography (CTC) for imaging the large bowel, not having the disadvantage of radiation exposure.
| Magnetic Resonance Imaging (MRI)
An MRI (Magnetic Resonance Imaging) scan uses magnetism to build up cross-sectional pictures of the body.
MRI of rectal cancers is currently proposed as a technique for pre-operative staging of rectal cancers and as a technique for re-imaging cancers following pre-operative radiotherapy.
Before the scan, the patient may be given an injection of dye into a vein in the arm, to improve the image.
The scan takes about 30 minutes, during which time the patient will lie inside a chamber which is often long and narrow.
This can feel claustrophobic.
People who have heart monitors, pacemakers or certain types of surgical clips cannot have an MRI because of the magnetic fields.
MRI of rectal cancers is currently proposed as a technique for pre-operative staging of rectal cancers and as a technique for re-imaging cancers following pre-operative radiotherapy.
Before the scan, the patient may be given an injection of dye into a vein in the arm, to improve the image.
The scan takes about 30 minutes, during which time the patient will lie inside a chamber which is often long and narrow.
This can feel claustrophobic.
People who have heart monitors, pacemakers or certain types of surgical clips cannot have an MRI because of the magnetic fields.
| Sigmoidoscopy
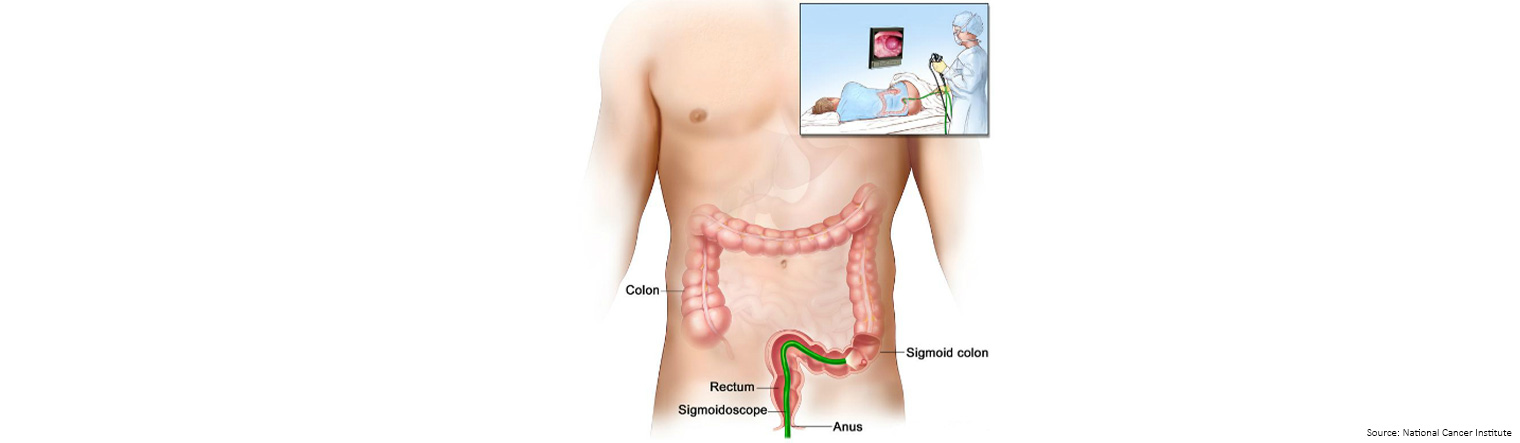
Sigmoidoscopy is a procedure to look inside the rectum and sigmoid (lower) colon (the first 60cm of the bowel) for polyps, abnormal areas, or cancer.
A sigmoidoscope is inserted through the anus and rectum into the lower part of the colon (sigmoid colon).
A sigmoidoscope is a thin, tube-like instrument with a light and a lens for viewing.
It may also have a tool to remove polyps or tissue samples, which are checked under a microscope for signs of cancer
A sigmoidoscope is inserted through the anus and rectum into the lower part of the colon (sigmoid colon).
A sigmoidoscope is a thin, tube-like instrument with a light and a lens for viewing.
It may also have a tool to remove polyps or tissue samples, which are checked under a microscope for signs of cancer
 | Barium enema
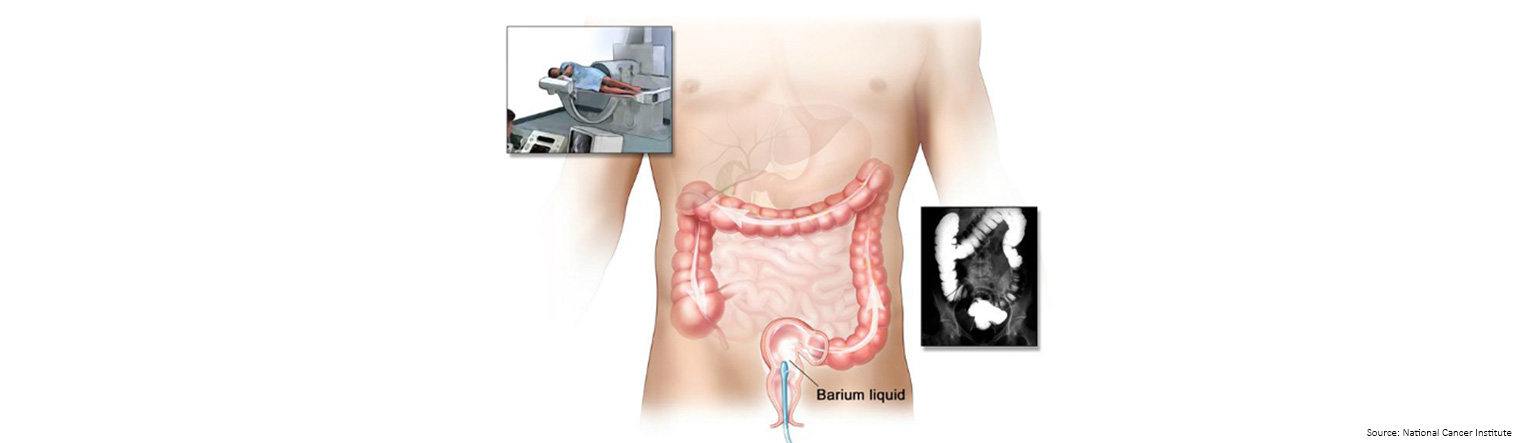
| Barium enemaA barium enema is a series of x-rays of the lower gastrointestinal tract.
The patient lies on an x-ray table and a liquid that contains barium (a silver-white metallic compound) is put into the rectum.
The barium flows through the colon and coats the lower gastrointestinal tract.
X-rays are taken to look for abnormal areas.
Any abnormal areas show up as black against the white liquid.
This procedure is also known as a lower gastrointestinal (GI) series.
The patient lies on an x-ray table and a liquid that contains barium (a silver-white metallic compound) is put into the rectum.
The barium flows through the colon and coats the lower gastrointestinal tract.
X-rays are taken to look for abnormal areas.
Any abnormal areas show up as black against the white liquid.
This procedure is also known as a lower gastrointestinal (GI) series.
 | Faecal immunochemical test (FIT)
| Faecal immunochemical test (FIT)The faecal immunochemical test (FIT) is a screening test for bowel cancer that can be completed in the privacy of your home. It tests for non-visible blood in poo, which can be an early sign of bowel cancer. FIT only detects human blood from the lower intestines. Medicines and foods do not interfere with the test.
FIT has been selected as the preferred testing method for Bowel Cancer Australia's BowelScreen Australia program and the tax-payer funded National Bowel Cancer Screening Program (NBCSP).
False-negative test results can occur
Screening test results may appear to be normal even though bowel cancer is present. A person who receives a false-negative test result (one that shows there is no blood in poo when there really is) may delay seeking medical care.
Screening test results may appear to be normal even though bowel cancer is present. A person who receives a false-negative test result (one that shows there is no blood in poo when there really is) may delay seeking medical care.
False-positive test results can occur
Screening test results may appear to be abnormal even though no blood in poo is present. A false-positive test result (one that shows there is blood in poo when there really isn't) can cause anxiety and is usually followed by more tests (such as colonoscopy), which also have risks.
Screening test results may appear to be abnormal even though no blood in poo is present. A false-positive test result (one that shows there is blood in poo when there really isn't) can cause anxiety and is usually followed by more tests (such as colonoscopy), which also have risks.
Visit Bowel Cancer Australia's screening webpage for more information.

| Stool DNA test
In August 2014, the US Food and Drug Administration (FDA) approved Cologuard, the first poo-based bowel cancer screening test that detects the presence of red blood cells and DNA mutations that may indicate the presence of certain kinds of abnormal growths that may be cancers such as bowel cancer or precursors to cancer.
Using a poo sample, Cologuard detects hemoglobin, a protein molecule that is a component of blood. Cologuard also detects certain mutations associated with bowel cancer in the DNA of cells shed by advanced adenomas as poo moves through the large intestine and rectum. Patients with positive test results are advised to undergo a diagnostic colonoscopy.
In 2019, the FDA approved the expansion of the age range for Cologuard from 50 years or older to 45 years or older.

| Blood tests
Colvera
In 2016, the CSIRO announced that a new, more accurate blood test to detect bowel cancer recurrence, known as Colvera, had launched in the United States.
The blood test is the result of a collaboration between CSIRO, Flinders University and Clinical Genomics.
According to CSIRO, bowel cancer usually recurs in the first two to three years following initial diagnosis and treatment, in 30-50 percent of cases.
The current method of monitoring for recurrence is through a blood test for CEA (carcinoembryonic antigen), together with CT scans and other clinical assessments.
“By providing clinicians with a new blood test that is more sensitive for recurrence than CEA, Colvera increases the likelihood of detecting curable recurrences of bowel cancer, with the ultimate aim of saving lives,” CSIRO Scientist Dr Trevor Lockett said.
According to Professor Graeme Young of Flinders Centre for Innovation in Cancer said, “Our study has shown that Colvera is significantly more sensitive for bowel cancer than CEA and as such provides us with an improved, simple test that increases the likelihood of detecting curable recurrence.”
The blood test is the result of a collaboration between CSIRO, Flinders University and Clinical Genomics.
According to CSIRO, bowel cancer usually recurs in the first two to three years following initial diagnosis and treatment, in 30-50 percent of cases.
The current method of monitoring for recurrence is through a blood test for CEA (carcinoembryonic antigen), together with CT scans and other clinical assessments.
“By providing clinicians with a new blood test that is more sensitive for recurrence than CEA, Colvera increases the likelihood of detecting curable recurrences of bowel cancer, with the ultimate aim of saving lives,” CSIRO Scientist Dr Trevor Lockett said.
According to Professor Graeme Young of Flinders Centre for Innovation in Cancer said, “Our study has shown that Colvera is significantly more sensitive for bowel cancer than CEA and as such provides us with an improved, simple test that increases the likelihood of detecting curable recurrence.”
Clinical trials have shown Colvera to be more than twice as sensitive for bowel cancer recurrence as the current CEA test.
The blood test is not currently available in Australia.
ColoSTAT
Rhythm Biosciences is developing a ColoSTAT blood test that measures the presence/concentration of multiple protein biomarkers for bowel cancer.
When certain combinations of protein biomarkers are measured in a blood sample, and concentrations weighted using an algorithm, a bowel cancer risk score can be determined. It is this patented process that forms the basis for the ColoSTAT technology.
Rhythm Biosciences is now working towards turning this technology into an in-market product.